Our Pipeline
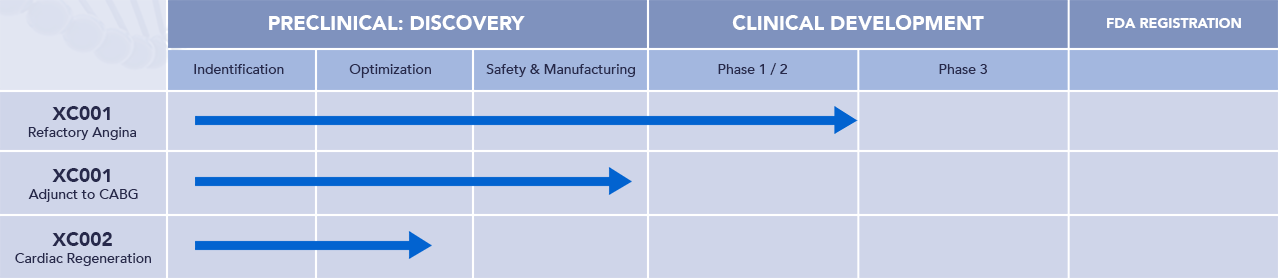
XC001: Refractory Angina – EXACT 1 is Complete, EXACT 2 to Commence in 2025
XC001 is an investigational gene therapy for the treatment of patients with refractory angina with no treatment options. Patients who have exhausted pharmacologic options and are not eligible for PCI or coronary artery bypass grafts become sedentary because of their symptoms and this can exacerbate comorbidities causing rapid deterioration of their health status and results in a poor quality of life because of the associated chest pain caused by the ischemia when even moderate physical exertion occurs. XC001 is designed to stimulate the formation of new coronary blood vessels to serve areas of the heart that are not receiving adequate blood supply. This treatment strategy is based on both pre-clinical and clinical evidence and should enable the patients to become less limited in their daily activities because of debilitating chest pain. An Investigational New Drug (IND) application for XC001 is open with the FDA. Enrollment in the Refractory Angina trial is complete.
XC001: Adjunct to CABG – Commencing in 2025
Coronary Artery Bypass Graft Surgery or CABG is a procedure used to treat coronary artery disease – the narrowing or blockage of the blood vessels that supply oxygen and nutrients to the heart muscle. During CABG, a healthy artery or vein from the body is connected, or grafted, to the blocked coronary artery. The grafted artery or vein bypasses the blocked portion of the coronary artery. This creates a new passage, and oxygen‑rich blood is routed around the blockage to the heart muscle. Approximately 500,000 CABG procedures are performed annually in the United States, in which an estimated one‑third of patients are at risk for incomplete coronary revascularization, often resulting in persistent angina. An adjunctive treatment to CABG, such as gene therapy with XC001, may reduce the incidence of incomplete revascularization.
XC002 – Discovery stage
XC002 is the second XyloCor program and it is being developed for regeneration of cardiac tissue in patients who have poorly functioning hearts as a result of past heart attacks and resulting heart failure. XC002 has transformed non-functioning cardiac cells into function cardiac cells in animal models.
Expanded Access Policy
XyloCor Therapeutics focuses on developing novel gene therapy products for unmet needs in advanced cardiovascular diseases. The following is XyloCor Therapeutics’ global expanded access policy (EAP).
Per the U.S. Food and Drug Administration (FDA) website, “expanded access is a potential pathway for a patient with a serious or immediately life‑threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.”
A number of factors consistent with the policies of the FDA and guidelines of international regulatory agencies should be taken into account when considering expanded access. They include:
- The illness must be serious or life‑threatening
- There are no other viable treatment options (such as approved products or enrolling clinical trials)
- There is sufficient evidence that the potential benefit to the patient would likely outweigh the potential risks based on safety and efficacy information available at the time
- Ability to provide a product in a fair and equitable manner, so that there is adequate manufacturing capacity for ongoing programs
- Whether granting expanded access would potentially compromise the scientific validity of broader development programs, or interfere with or delay the conduct of current clinical trials or regulatory submissions designed to make the product candidate available to many more patients
At this time, XyloCor Therapeutics believes that participation in a clinical trial is the best way to receive our investigational product. Xylocor does not currently have any investigational gene therapy product candidates available for expanded access. If you have questions about our investigational product candidates or expanded access, please submit them to info@XyloCor.com.
In the event that XyloCor Therapeutics decides to make its investigational product candidates available on an expanded access basis, this policy will be updated with a hyperlink to the expanded access record on clinicaltrials.gov after such record becomes active.
Requests for expanded access to XyloCor Therapeutics’ investigational gene therapy product candidate must come from the patient’s treating physician. If XyloCor Therapeutics decides to make an investigational gene therapy product available through an EAP, XyloCor Therapeutics will evaluate and respond to each expanded access request that it receives on a case‑by‑case basis using criteria that ensures such requests are considered in a consistent manner. XyloCor Therapeutics will strive to acknowledge receipt of any expanded access questions or requests within ten business days of receipt.
The posting of this policy by XyloCor Therapeutics shall not serve as a guarantee of access to any specific investigational drug by any individual patient. As authorized by and in accordance with the 21st Century Cures Act, XyloCor Therapeutics may revise this posted expanded access policy at any time.